Multiple thermal magnetic relaxation in a two-dimensional ferromagnetic dysprosium(iii) metal–organic framework†
Abstract
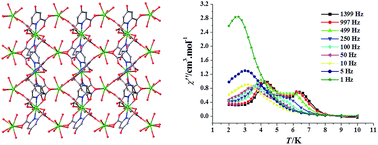
A new two-dimensional (2D) lanthanide metal–organic framework, {[Dy2(HCAM)3(H2O)4]·2H2O}n (1, H3CAM = 4-hydroxypyridine-2,6-dicarboxylic acid) has been hydrothermally synthesized and structurally characterized using single-crystal X-ray diffraction. There are two crystallographically independent dysprosium atoms in 1, displaying spherical tricapped trigonal prism geometry and square antiprism geometry, respectively. The dysprosium(III) ions are connected with each other through the bridging HCAM2− anions, generating a classical 2D (4, 4) grid structure. Magnetic investigations revealed that intramolecular ferromagnetic interactions exist among the dysprosium(III) ions in 1, which shows field-induced two-step thermal magnetic relaxation, with the effective thermal barriers of 63.5 K and 57.1 K, respectively.


 Please wait while we load your content...
Please wait while we load your content...