The biorelevant concentration of Tween 80 solution is a simple alternative medium to simulated fasted state intestinal fluid†
Abstract
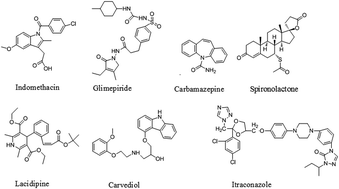
The aim of the present study is to investigate whether the use of the biorelevant concentration of conventional surfactants as an alternative medium to simulated fasted state intestinal fluid (FaSSIF) for drugs with different acid–base properties is feasible. First, the equilibrium solubility of seven drugs representing diverse acid–base properties was determined. The solubility of those drugs was studied in pH 6.5 blank FaSSIF, FaSSIF containing physiological levels of sodium taurocholate and lecithin, and blank FaSSIF containing a series of concentrations of Tween 80 or sodium dodecyl sulfate (SDS) at 37 °C using the shake-flask method. The results demonstrated that the solubility of the seven drugs in 0.07% and 0.10% Tween 80 solutions matched better with the biorelevant media FaSSIF than SDS solutions (0.05% to 0.10%). Then the dissolution behavior of the BCS class II drug lacidipine was determined in SDS solutions, Tween 80 solutions and FaSSIF. Compared with FaSSIF, 0.07% Tween 80 was proved to be the best biorelevant-mimetic medium for lacidipine oral solid formulations. Then the dissolution tests of micronized lacidipine, carbamazepine, glimepiride and carvedilol tablets were conducted as internal and external validations, respectively. The results all demonstrated that the biorelevant concentration of Tween 80 could be set as 0.07% and such a cost-effective medium will hold a great potential in drug development and quality control of oral solid dosage forms.

 Please wait while we load your content...
Please wait while we load your content...