Copper promoted synthesis of substituted quinolines from benzylic azides and alkynes†
Abstract
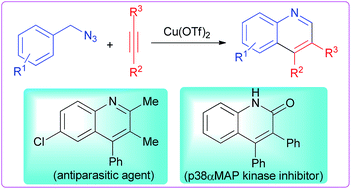
A novel copper promoted synthesis of substituted quinolines from various benzylic azides and internal alkynes has been demonstrated. The reaction features a broad substrate scope, high product yields and excellent regioselectivity. In contrast to the known two-step process of acid promoted [4 + 2] cycloaddition reaction and oxidation, the present methodology allows the synthesis of quinolines in a single step under neutral reaction conditions and can be applied to the synthesis of biologically active 6-chloro-2,3-dimethyl-4-phenylquinoline (antiparasitic agent) and 3,4-diphenylquinolin-2(1H)-one (p38αMAP kinase inhibitor). A plausible reaction mechanism involves rearrangement of benzylic azide to N-arylimine (Schmidt reaction) followed by intermolecular [4 + 2] cycloaddition with internal alkynes.

 Please wait while we load your content...
Please wait while we load your content...