Reactant cum solvent water: generation of transient λ3-hypervalent iodine, its reactivity, mechanism and broad application†
Abstract
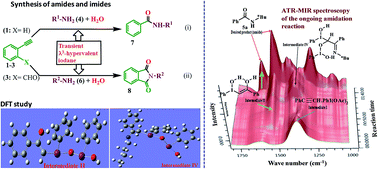
An outstanding amidation/imidation process is demonstrated under metal-free benign reaction conditions by grafting terminal alkynes with varied amines and reactant cum solvent water. We explored diverse substrate scope in the nonconventional approach for synthesis of several classes of valuable compounds bearing amide linkages such as N-tosyl, N-aminotosyl, N-oxobenzyl, N-alkylated sugar-based secondary and tertiary chiral amides and also exploiting it for synthesis of valuable cyclic imides through simultaneous activation of triple bond and sp2C–H of aldehyde. The in situ-generated powerful reagent PhI(OH)2 enables selective cleavage and functionalization of terminal C–C triple bonds through simultaneous construction of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. A measurable breakthrough in time-resolved ATR-MIR spectroscopy of the ongoing λ3-hypervalent iodine controlled reaction revealed formation of the unknown intermediates. The ATR-MIR technology is used for identifying infrared (IR) spectra of the individual intermediate/component present in the reaction mixture, which contains several compounds. The structures of the intermediates and their IR spectra were determined by DFT study. This unprecedented combination of experimental results and theoretical prediction provided useful information regarding the reaction insights such as the cleavage of triple bond, amination and amidation in the complex reaction process involving λ3-hypervalent iodine-bearing labile intermediates to the desired amide. The NMR and labelling (2H and 18O) studies supported the DFT-IR predicted reaction pathway.
O bonds. A measurable breakthrough in time-resolved ATR-MIR spectroscopy of the ongoing λ3-hypervalent iodine controlled reaction revealed formation of the unknown intermediates. The ATR-MIR technology is used for identifying infrared (IR) spectra of the individual intermediate/component present in the reaction mixture, which contains several compounds. The structures of the intermediates and their IR spectra were determined by DFT study. This unprecedented combination of experimental results and theoretical prediction provided useful information regarding the reaction insights such as the cleavage of triple bond, amination and amidation in the complex reaction process involving λ3-hypervalent iodine-bearing labile intermediates to the desired amide. The NMR and labelling (2H and 18O) studies supported the DFT-IR predicted reaction pathway.

 Please wait while we load your content...
Please wait while we load your content...