Effects of terminal group and chain length on temperature-responsive chromatography utilizing poly(N-isopropylacrylamide) synthesized via RAFT polymerization†
Abstract
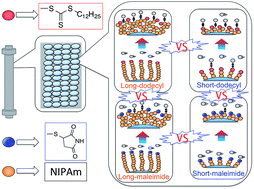
Poly(N-isopropylacrylamide) with two chain lengths (MW: ca. 5000, and ca. 20 000 g mol−1) was synthesized by reversible addition-fragmentation chain transfer (RAFT) polymerization, using 2-dodecylsulfanylthiocarbonylsulfanyl-2-methyl propionic acid as the chain transfer agent. The derivatization of terminal dodecyl groups to maleimide groups was performed through the reduction of terminal trithiocarbonate moieties. These polymers were grafted onto aminopropyl silica using an activated ester–amine coupling method. Five types of polymer modified silica beads were prepared, i.e., long chain length, short chain length, and mixed chain length functionalized with a dodecyl terminal group, and long chain length and short chain length with a maleimide terminal group. The surface properties of the modified polymer silica beads were evaluated by employing them as the stationary phase in HPLC setups and examining the temperature-dependent elution profiles of five steroids. The retention factor of steroids became smaller when the terminal dodecyl group was substituted with a maleimide group. A hydrophobic dodecyl moiety on the outermost surface strongly affected the retention of steroids, and the retention factor of steroids on short chains was larger than that on long chains below the lower critical solution temperature. These results indicate that the chain length and terminal functional group on the outermost surface of the polymer have a critical effect on the characteristics of polymer modified silica bead interfaces.

 Please wait while we load your content...
Please wait while we load your content...