Synthesis of functionalized isoxazole–oxindole hybrids via on water, catalyst free vinylogous Henry and 1,6-Michael addition reactions†
Abstract
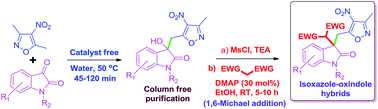
Various 3-substituted-3-hydroxy isoxazole–oxindole hybrids were synthesized via the vinylogous Henry reaction of 3,5-dimethyl-4-nitroisoxazole and isatin using water as a reaction medium under catalyst free conditions at 50 °C. Systematic studies were carried out to understand the role of the water on the reaction by using D2O, brine, ethylene glycol and PEG-400 as a reaction medium along with organic solvents. Among all of these, water (0.170 mol concentration) was found be more efficient giving desired products in 82–99% yields in 45–120 min. Further the quaternary centre (3° alcohol) generated was used for the creation of a double bond which was again used for a 1,6-Michael reaction to produce highly functionalized isoxazole–oxindole derivatives.

 Please wait while we load your content...
Please wait while we load your content...