Synthesis and characterization of a novel, ditopic, reversible and highly selective, “Turn-On” fluorescent chemosensor for Al3+ ion†
Abstract
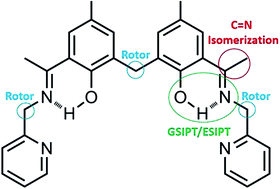
We herein report a structurally characterized Schiff base ligand, L, formed by the condensation of 1,1-bis-[2-hydroxy-3-acetyl-5-methylphenyl]methane with 2-picolyl amine. It utilizes the three signalling mechanisms, ESIPT, chelation enhanced fluorescence (CHEF) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization, to serve as a “Turn-On” fluorescence chemosensor for Al3+. L has high selectivity for Al3+ in MeOH. The reversible nature of this chemosensor makes it cost effective. It joins the rare family of ditopic fluorescent chemosensors. When this Schiff base receptor was treated with Al3+ salt in MeOH, the fluorescence intensity abruptly increased. Other metal ions did not show such a significant effect on the fluorescence. The detection limit for this chemosensor was found to be 0.7 μM.
N isomerization, to serve as a “Turn-On” fluorescence chemosensor for Al3+. L has high selectivity for Al3+ in MeOH. The reversible nature of this chemosensor makes it cost effective. It joins the rare family of ditopic fluorescent chemosensors. When this Schiff base receptor was treated with Al3+ salt in MeOH, the fluorescence intensity abruptly increased. Other metal ions did not show such a significant effect on the fluorescence. The detection limit for this chemosensor was found to be 0.7 μM.

 Please wait while we load your content...
Please wait while we load your content...