Formation of cyclobutane thymine dimers by tiaprofenic acid and its photoproducts: approach to the photosensitizer triplet state energy limit value
Abstract
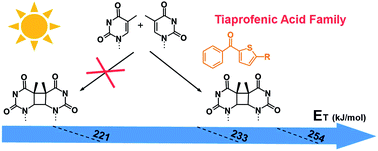
Cyclobutane thymine dimers, the major photoproducts produced in UV-irradiated DNA, are the main causative agents for mutagenesis and skin cancer. This lesion can also be initiated under UVA radiation, involving triplet–triplet energy transfer mechanism from a photosensitizer to the thymine nucleobase. According to previous reports, only photosensitizers with a triplet state energy >270 kJ mol−1 should be able to induce cyclobutane thymine dimers photosensitization. However, tiaprofenic acid, a non-steroidal anti-inflammatory drug widely prescribed in the treatment of inflammation and pain, has shown cyclobutane thymine dimers photosensitization, although its triplet energy state value and those of its photoproducts are lower than the one previously reported for thymine in DNA. In this context, the in vitro photosensitizing properties of tiaprofenic acid and its photoproducts were studied by agarose gel electrophoresis and phosphorescence experiments and demonstrated clearly the formation of cyclobutane thymine dimers by tiaprofenic acid and its photoproducts. This study allows us to approach the lower limit threshold of the triplet state energy of a photosensitizer for cyclobutane thymine dimers formation and thereby to improve the prediction of the photogenotoxic potential of current and future drugs.

 Please wait while we load your content...
Please wait while we load your content...