Direct synthesis of butadiynyl-substituted pyrroles under solvent- and transition metal-free conditions†
Abstract
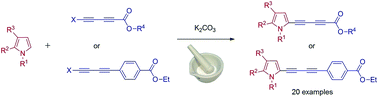
The work describes a convenient and highly efficient C–H butadiynylation of substituted pyrroles with the use of 1-halobutadiynes. The method requires only a simple grinding of substrates in a mortar under mild, solvent- and transition metal-free conditions and constitutes the first example of pyrrole butadiynylation via cross-coupling reaction with the use of 1-halobutadiynes. The scope of this mechanochemical approach covers 4,5,6,7-tetrahydro-1H-indole, its N-substituted derivatives and 2-phenylpyrrole and on the other hand ester and phenyl end-capped 1-halobutadiynes including chlorides, bromides and iodides. Interestingly, the method has proven effective also for weak electron withdrawing aryl substituted 1-halobutadiynes what has not been yet achieved for 1-haloacetylenes. Such reactivity was unexpected in the view of the literature data and opened a gate to the plethora of substrates for organic synthesis including syntheses of pharmaceuticals. An X-ray analysis of two coupling products is also presented.

 Please wait while we load your content...
Please wait while we load your content...