Efficient catalytic activation of Suzuki–Miyaura C–C coupling reactions with recyclable palladium nanoparticles tailored with sterically demanding di-n-alkyl sulfides†
Abstract
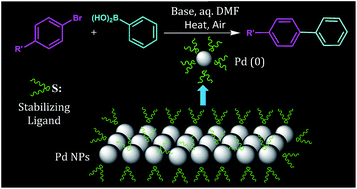
n-Bromodocosane reacts with Na2S, generated in situ by the reduction of elemental sulfur with NaBH4, to give n-didocosyl sulfide (L1), which acts as a protector for palladium nanoparticles (2–7) that are prepared using different palladium precursors in the presence of L1 (Pd : L1 ratio 1 : 2 and 4 : 1). The NPs have been characterized with powder X-ray diffraction, SEM, EDX, UV-vis spectroscopy and HRTEM. The size (nm) ranges for the majority of spherical NPs 2–7 are ∼18–19, 4–5, 5–7, 4–6, 7–9 and 4–6 respectively. The precursor of palladium affects the size, shape and dispersion of the NPs. When [Pd(CH3CN)2Cl2]/Na2PdCl4 was used as a precursor, uniformly dispersed NPs of narrow size range were obtained. L1 and its complex [Pd(L1)2Cl2] (1) have also been synthesized by the reaction of Na2PdCl4 with L1 and characterized with 1H and 13C{1H} NMR spectroscopy. The NPs show good catalytic activity for the Suzuki–Miyaura coupling (SMC) of various aryl chlorides/bromides with phenylboronic acid at low catalyst loading (0.1–0.5 mol% of Pd). The conversion is good for some aryl halides in a short reaction time of the order 1–2 h. Among 2–7, the highest activity is observed for Pd NPs obtained from Na2PdCl4, which is probably due to uniformity in their size and dispersion. The distinct advantage of NPs 2–7 is that they can be separated and reused at least up to five times. The complex 1, equivalent to 0.001 mol% Pd, is efficient for the SMC of some aryl halides, as good conversion into coupled products has been observed. Two phase tests, conducted for 1 and 3, suggest the contribution of both homogeneous and heterogeneous catalytic pathways in overall catalysis.

 Please wait while we load your content...
Please wait while we load your content...