Conversion of chitin and N-acetyl-d-glucosamine into a N-containing furan derivative in ionic liquids†
Abstract
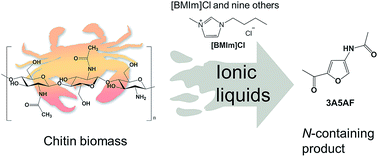
Direct conversion of chitin to 3-acetamido-5-acetylfuran (3A5AF) in a range of ionic liquids (ILs) has been systematically investigated. 10 ILs with different cations and anions were tested as the solvent and 25 additives were screened. The results revealed that the presence of Cl in the IL is essential. In addition to the solubility enhancement of chitin in Cl containing ILs, the Cl anion appeared to participate in the chitin reaction cycle in IL solvents. 3A5AF can be obtained in some ILs, such as [BMIm]Cl, without any additive. Significantly enhanced yields of 3A5AF were obtained in [BMIm]Cl using boric acid and hydrochloric acid (HCl) as additives at 180 °C, a lower temperature than using organic solvents (215 °C). Kinetic studies showed that the product formed very quickly within 10 min, with much higher initial rate than using organic solvents. Recovered chitin was characterized by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and elemental analysis (EA). In an effort to improve the yield, extraction and distillation were attempted for both chitin and chitin monomer, N-acetyl-D-glucosamine (NAG). Further studies were performed on NAG to see if acidic ILs would lead to enhanced reactivity. However, these were less effective.


 Please wait while we load your content...
Please wait while we load your content...