Riboflavin detection by α-Fe2O3/MWCNT/AuNPs-based composite and a study of the interaction of riboflavin with DNA†
Abstract
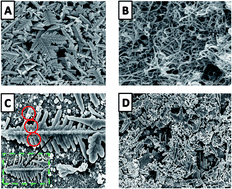
The electrochemical behavior of riboflavin (RF) at a glassy carbon electrode modified with α-Fe2O3/MWCNT/AuNPs was investigated by cyclic voltammetry (CV) and square wave voltammetry (SWV). The redox behavior of the RF was examined in detail in phosphate buffer solution using variable scan rate cyclic voltammetry (V = 20 mV s−1–10 V s−1) and has been found to undergo a series of proton-coupled electron transfer reactions. Moreover, the interaction of RF with double stranded DNA (ds-DNA) based on the oxidation signal of the guanine base was studied using SWV. Our sensor fabrication did not take into account the pretreatment procedure of the modified electrode to immobilize the ds-DNA, which shows that our composite material has highly electroactive and many functional groups to interact with ds-DNA. The decrease in the intensity of the guanine oxidation signal after interaction with RF was found as an indicator signal for the sensitive determination of RF. These results additionally confirmed that this RF–ds-DNA interaction could be used for sensitive and accurate detection of RF. The peak current on the modified electrode was linear over a range from 0.3 μM to 0.6 × 10−8 M, with the detection limit of 6 nM (3σ/b). The analytical performance of this sensor was demonstrated in real samples with satisfactory recovery.

 Please wait while we load your content...
Please wait while we load your content...