Supported gold-catalyzed and ammonia-promoted selective synthesis of quinazolines in aqueous media†
Abstract
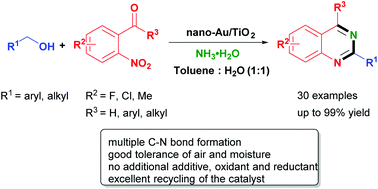
A highly efficient and selective nitrogen source-promoted reaction for the synthesis of 2,4-disubstituted quinazolines from o-nitroacetophenones and alcohols catalyzed by Au/TiO2 has been developed via a hydrogen-transfer strategy. This reaction has good tolerance to air and water, a wide substrate scope, and represents a new avenue for practical multiple C–N bond formation. More importantly, no additional additive, oxidant and reductant are required in the reaction and the catalyst can be recovered and reused readily.

 Please wait while we load your content...
Please wait while we load your content...