Synthesis of covalent triazine-based frameworks with high CO2 adsorption and selectivity†
Abstract
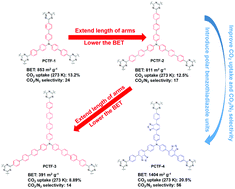
Four covalent triazine-based frameworks (PCTF-1 to PCTF-4) with triphenylamine as the core were synthesized by a consolidated ionothermal reaction between aromatic nitriles under the catalysis of ZnCl2. The Brunauer–Emmett–Teller (BET) specific surface area values of PCTF-1 (853 m2 g−1), PCTF-2 (811 m2 g−1) and PCTF-3 (391 m2 g−1) are gradually decreased when increasing the length of branched arm. It indicates that PCTF using monomers with longer branches can be packed more efficiently, resulting in higher density and lower surface area. PCTF-4, compared with PCTF-2, just has the middle benzene of the branches replaced with benzothiadiazole, however N2 adsorption isotherms showed that its BET specific surface area value (1404 m2 g−1) is the highest among the PCTFs, almost two times that of PCTF-2. The nitrogen-rich characteristics of C3N3 triazine rings result in the frameworks’ strong affinity for CO2 and thereby high CO2 adsorption capacity. In particular PCTF-4 with benzothiadiazole exhibited the highest CO2 uptake (20.5 wt%) at 273 K and 1 bar, this value is one of the highest reported values for covalent triazine-based frameworks. The results demonstrate that the introduction of strong polar groups (benzothiadiazole) into a polymer skeleton is an efficient strategy to produce CO2-philic microporous organic polymers with enhanced binding affinity to CO2 molecules. In addition, such PCTFs with high physical–chemical stability and comparable BET surface areas exhibited ideal CO2/N2 selectivities (14–56) and CO2/CH4 selectivities (11–20) at 273 K, showing that these materials are potential candidates for gas storage and separation. However, in the presence of water vapor, the CO2 uptake of all polymers decreased probably due to the formation of hydrogen bonds with water, suggesting that materials that perform well under dry conditions may deteriorate under practical conditions.

 Please wait while we load your content...
Please wait while we load your content...