A poly(polyoxometalate)-b-poly(hexanoic acid) block copolymer: synthesis, self-assembled micelles and catalytic activity†
Abstract
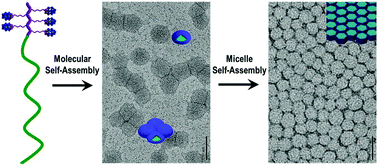
A hybrid block copolymer (H-BCP) composed of a poly(polyoxometalate) (poly(POM)) block and a poly(6-norbornene-hexanoic acid) (poly(COOH)) block was prepared by sequential addition of two norbornene monomers containing a POM cluster and a carboxyl as side groups via ring-opening metathesis polymerization (ROMP). Because the 5833 Da molecular weight of the POM-containing monomer is much higher than 277 Da of the carboxyl-containing monomer, the designed H-BCP has a degree of polymerization DP = 5 for the poly(POM) block and DP = 200 for the poly(COOH) block. Thus, the H-BCP not only has differences in its chemical structure and properties but also asymmetries in its molecular weight and length. In acetonitrile, it self-assembles into hybrid micelles with a thin shell of the poly(POM) block and a core of the poly(COOH) block. The micelles form thin films in which the micelles pack into a hexagonal pattern driven by attractive capillary forces between the micelles. Meanwhile, the H-BCP exhibits good catalytic activity. The H-BCP may become an important candidate for producing hybrid materials with good processability and ductility of the polymer components, and good functionality of the POM clusters.

 Please wait while we load your content...
Please wait while we load your content...