A rather facile strategy for the fabrication of PEGylated AIE nanoprobes†
Abstract
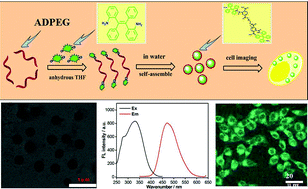
Fluorescent organic nanoparticles (FNPs) have attracted great research interest for biological sensors, biological imaging and disease treatment. However, the preparation of ultrabright FNPs using conventional organic dyes is still a challenge for their aggregation caused quenching effect. In this work, we reported for the first time that polyethylene glycol (PEG) and an aggregation induced emission (AIE) dye, 2,2′-diaminotetraphenyl ethylene (DATPE), can be facilely conjugated by trimellitic anhydride chloride. Taking advantage of the different reaction activities of anhydride and chloride, anhydride-terminated PEG (ADPEG) was first synthesized through the reaction between hydroxyl groups and benzoyl chloride. Then ADPEG could further react with the amino groups of DATPE. Because of the AIE properties of DATPE, these amphiphilic triblock copolymers can self-assemble into FNPs (PEG-TPE FNPs) and emit strong blue-green fluorescence in aqueous solution. Cell uptake behavior and cytotoxicity evaluation suggested that PEG-TPE FNPs possessed excellent cytocompatibility and could be facilely taken up by cells, implying that PEG-TPE FNPs are promising for biomedical applications. More importantly, the method described in this work is rather simple and effective and, more importantly, can be extended to fabricate many other multifunctional FNPs on a large scale for various biomedical applications.

 Please wait while we load your content...
Please wait while we load your content...