Side chain liquid crystalline polymers with an optically active polynorbornene backbone and achiral mesogenic side groups†
Abstract
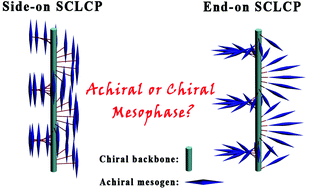
Most of the traditional chiral side-chain liquid crystalline polymers (SCLCPs) depend on pendant chiral mesogenic units to introduce chirality into their structure, with the polymer backbones being usually achiral. In this work, we asymmetrically synthesize several enantiomerically pure norbornene monomers functionalized with achiral mesogenic units, and further apply a ring-opening metathesis polymerization technique to prepare a series of side-on and end-on SCLCPs with an optically active polynorbornene main chain and achiral mesogens. Their physical properties are fully characterized by NMR, UV, CD, GPC, TGA, DSC, polarimetry, polarized optical microscopy and small-angle X-ray scattering. The obtained side-on SCLCPs display the tendency to form nematic, i.e. achiral mesophases, in strong contrast to the chiral nematic (cholesteric) mesophase exhibited by their comparative end-on analogues. The proposed explanation for this phenomenon is that the chiral backbones and the laterally attached mesogens of side-on SCLCPs can concurrently exist in a parallel arrangement so that the mesogenic directors might not be affected by the chirality information, while the mesogenic directors of end-on SCLCPs always tilt to the backbone orientation so that the twisting power of chiral main chains might force the terminally attached mesogens to form helical structures.

 Please wait while we load your content...
Please wait while we load your content...