Synthesis of well-defined α,ω-telechelic multiblock copolymers in aqueous medium: in situ generation of α,ω-diols†
Abstract
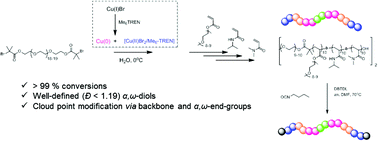
The synthesis of well-defined α,ω-dihydroxyl telechelic multiblock copolymers by sequential in situ chain extensions via aqueous Cu(0) mediated living radical polymerization (SET-LRP) is reported. The rapid disproportionation of Cu(I)Br in the presence of Me6-TREN in water has been exploited to generate Cu(0) and [Cu(II)Br2/Me6-TREN] in situ, resulting in rapid reaction rate and narrow molecular weight distributions. Under optimized conditions, a telechelic heptablock copolymer was obtained within 2 hours with a final dispersity of ∼1.1 while the monomer conversion was >99% for each block. A range of acrylamides and acrylates have been successfully incorporated within the same polymer backbone, including N-isopropylacrylamide (NIPAAm), N,N-diethylacrylamide (DEA) and N,N-dimethylacrylamide (DMA) and poly(ethylene glycol) methyl ether acrylate (PEGA480). The thermo-responsive nature of these materials was subsequently demonstrated via cloud point measurements as both a function of molecular weight and backbone functionality. In addition, the typically unwanted hydrolysis of the α- and ω-end groups in aqueous media was further exploited via isocyanate post-polymerization modifications to alter the end group functionality.

 Please wait while we load your content...
Please wait while we load your content...