Ester-free thiol-X resins: new materials with enhanced mechanical behavior and solvent resistance†
Abstract
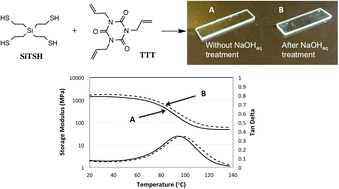
A series of thiol-Michael and radical thiol–ene network polymers were successfully prepared from ester-free as well as ester-containing monomer formulations. Polymerization reaction rates, dynamic mechanical analysis, and solvent resistance experiments were performed and compared between compositions with varied ester loading. The incorporation of ester-free alkyl thiol, vinyl sulfone and allylic monomers significantly improved the mechanical properties when compared with commercial, mercaptopropionate-based thiol–ene or thiol-Michael networks. For polymers with no hydrolytically degradable esters, glass transition temperatures (Tg's) as high as 100 °C were achieved. Importantly, solvent resistance tests demonstrated enhanced stability of ester-free formulations over PETMP-based polymers, especially in concentrated basic solutions. Kinetic analysis showed that glassy step-growth polymers are readily formed at ambient conditions with conversions reaching 80% and higher.

 Please wait while we load your content...
Please wait while we load your content...