On the photostability of scytonemin, analogues thereof and their monomeric counterparts†
Abstract
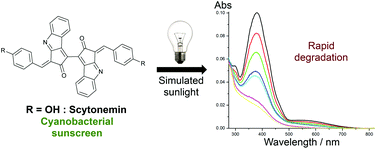
As a part of their sun-protective strategy, cyanobacteria produce the natural UV-screener scytonemin. Its accumulation in the extracellular sheaths allows the bacteria to thrive in inhospitable locations highly exposed to solar radiation. Scytonemin is often referred to as photostable and has been reported to be non-fluorescent. Taken together, these properties indicate inherently fast non-radiative relaxation processes. Despite these interesting traits, the photophysics of scytonemin is as yet almost completely unexplored. In this study, we have compared the steady-state photophysics of scytonemin itself and four derivatives thereof. Furthermore, the in vitro photostability of scytonemin was studied in different solvents using a solar simulation system. Scytonemin and the investigated derivatives demonstrated a more rapid photoinduced decay in comparison with two commercial UV-screening agents. The photostability could be modulated by varying the solvent, with the protic solvent ethanol providing the most stabilizing environment.

 Please wait while we load your content...
Please wait while we load your content...