Pyrimidinone: versatile Trojan horse in DNA photodamage?
Abstract
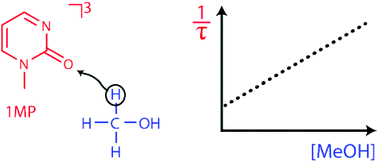
(6-4) Photolesions between adjacent pyrimidine DNA bases are prone to secondary photochemistry. It has been shown that singlet excited (6-4) moieties form Dewar valence isomers as well as triplet excitations. We here report on the triplet state of a minimal model for the (6-4) photolesion, 1-methyl-2(1H)-pyrimidinone. Emphasis is laid on its ability to abstract hydrogen atoms from alcohols and carbohydrates. Steady-state and time-resolved experiments consistently yield bimolecular rate constants of ∼104 M−1 s−1 for the hydrogen abstraction. The process also occurs intramolecularly as experiments on zebularine (1-(β-D-ribofuranosyl)-2(1H)-pyrimidinone) show.

 Please wait while we load your content...
Please wait while we load your content...