Supramolecular polymers for organocatalysis in water†
Abstract
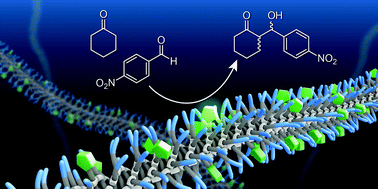
A water-soluble benzene-1,3,5-tricarboxamide (BTA) derivative that self-assembles into one-dimensional, helical, supramolecular polymers is functionalised at the periphery with one L-proline moiety. In water, the BTA-derivative forms micrometre long supramolecular polymers, which are stabilised by hydrophobic interactions and directional hydrogen bonds. Furthermore, we co-assemble a catalytically inactive, but structurally similar, BTA with the L-proline functionalised BTA to create co-polymers. This allows us to assess how the density of the L-proline units along the supramolecular polymer affects its activity and selectivity. Both the supramolecular polymers and co-polymers show high activity and selectivity as catalysts for the aldol reaction in water when using p-nitrobenzaldehyde and cyclohexanone as the substrates for the aldol reaction. After optimisation of the reaction conditions, a consistent conversion of 92 ± 7%, deanti of 92 ± 3%, and eeanti of 97 ± 1% are obtained with a concentration of L-proline as low as 1 mol%.

 Please wait while we load your content...
Please wait while we load your content...