A lactone-fused cyclohexadiene as a versatile platform for diversified synthesis of 5,6,5-tricyclic scaffolds†
Abstract
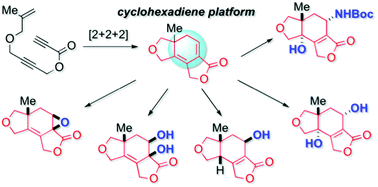
A lactone-fused cyclohexadiene, which can be readily prepared by ruthenium(III)-catalyzed [2 + 2 + 2] cyclization of an enediyne, functioned as a versatile platform for the stereoselective synthesis of differently functionalized 5,6,5-tricyclic scaffolds.

 Please wait while we load your content...
Please wait while we load your content...