A polystyrene-supported 9-amino(9-deoxy)epi quinine derivative for continuous flow asymmetric Michael reactions†
Abstract
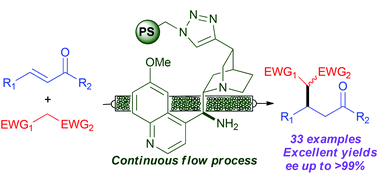
A polystyrene (PS)-supported 9-amino(9-deoxy)epi quinine derivative catalyzes Michael reactions affording excellent levels of conversion and enantioselectivity using different nucleophiles and structurally diverse enones. The highly recyclable, immobilized catalyst has been used to implement a single-pass, continuous flow process (residence time: 40 min) that can be operated for 21 hours without significant decrease in conversion and with improved enantioselectivity with respect to batch operation. The flow process has also been used for the sequential preparation of a small library of enantioenriched Michael adducts.
- This article is part of the themed collection: Recent Advances in Flow Synthesis and Continuous Processing

 Please wait while we load your content...
Please wait while we load your content...