Strategically designed biomodel: engineering C3–C4 cleavage of d-fructose†
Abstract
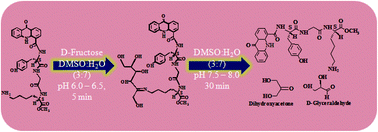
Amongst a library of aldolase inspired, rationally designed compounds, the acridine derivative carrying a (S)-Tyr-Gly-(S)-Lys tripeptide selectively effected C3–C4 scissoring of D-fructose and produced D-glyceraldehyde and dihydroxyacetone.

 Please wait while we load your content...
Please wait while we load your content...