An efficient transformation of furano-hydroxychalcones to furanoflavones via base mediated intramolecular tandem O-arylation and C–O bond cleavage: a new approach for the synthesis of furanoflavones†
Abstract
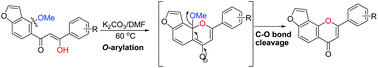
A new and efficient potassium carbonate mediated intramolecular tandem O-arylation followed by C–O bond cleavage of furano-hydroxychalcones is described. The treatment of furano-hydroxychalcones pongamol (1a) and ovalitenone (2a) with potassium carbonate in DMF led to the direct formation of the furanoflavones lanceolatin B (3ab) and pongaglabrone (4ab) in excellent yields. This is the first report on the cyclization of furano-hydroxychalcones via C–O bond cleavage (demethoxylation) to produce furanoflavonoids.

 Please wait while we load your content...
Please wait while we load your content...