Squaramide-catalysed asymmetric cascade aza-Michael/Michael addition reaction for the synthesis of chiral trisubstituted pyrrolidines†
Abstract
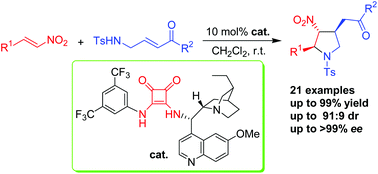
A bifunctional squaramide catalysed aza-Michael/Michael cascade reaction between nitroalkenes and tosylaminomethyl enones or enoates has been developed. This organocatalytic cascade reaction provides easy access to highly functionalized chiral pyrrolidines with a broad substrate scope, giving the desired products in good yields (up to 99%) with good diastereoselectivities (up to 91 : 9 dr) and excellent enantioselectivities (up to >99% ee) under mild conditions. This protocol provides a straightforward entry to highly functionalized chiral trisubstituted pyrrolidine derivatives from simple starting materials.

 Please wait while we load your content...
Please wait while we load your content...