Cross-dehydrogenative coupling of α-C(sp3)–H of ethers/alkanes with C(sp2)–H of heteroarenes under metal-free conditions†
Abstract
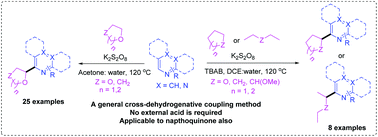
Here we have developed an effective metal-free dehydrogenative coupling method wherein α-oxyalkyl and alkyl radicals were generated from various ethers and alkanes to undergo coupling with a variety of electron-deficient heteroarenes such as un/substituted iso-quinolones, quinolines, pyridines, pyrazines and pyrimidines. The persulfate–acetone–water system was optimized for the dehydrogenative coupling with cyclic ethers which gave moderate to excellent yields of α-oxyalkyl containing heteroarenes. We have also optimized the conditions for coupling with cyclic alkanes and alicyclic ethers and demonstrated by conducting the reactions with a variety of electron-deficient heteroarenes. Further, the present method is also applicable to electron deficient arenes like naphthoquinones and moreover, it didn't require any external acid.

 Please wait while we load your content...
Please wait while we load your content...