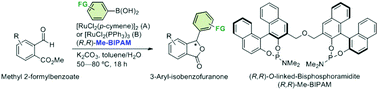
Enantioselective addition of arylboronic acids to methyl 2-formylbenzoates by using a ruthenium/Me-BIPAM catalyst for synthesis of chiral 3-aryl-isobenzofuranones†
Abstract
Ruthenium/Me-BIPAM-catalyzed asymmetric addition of arylboronic acids to methyl 2-formylbenzoates afforded chiral 3-aryl-isobenzofuranones. [RuCl2(p-cymene)]2/Me-BIPAM and RuCl2(PPh3)3/Me-BIPAM catalyst systems tolerate a variety of functional groups and give high yields with high enantioselectivities.

 Please wait while we load your content...
Please wait while we load your content...