Regioselective synthesis of nitrosoimidazoheterocycles using tert-butyl nitrite†
Abstract
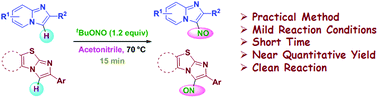
A simple and practical method has been developed for the regioselective nitrosylation of imidazopyridines via C(sp2)–H bond functionalization using tert-butyl nitrite under mild reaction conditions in a short time. A library of 3-nitrosoimidazopyridines with broad functionalities was synthesized in near quantitative yields. The present protocol is also applicable to imidazo[2,1-b]thiazole and benzo[d]imidazo[2,1-b]thiazole.

 Please wait while we load your content...
Please wait while we load your content...