Efficient one-pot synthesis of 5-perfluoroalkylpyrazoles by cyclization of hydrazone dianions†
Abstract
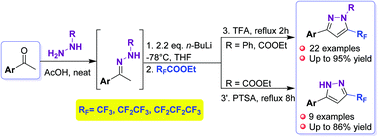
A highly selective and efficient method for the synthesis of 5-trifluoromethylated and 5-perfluoroalkylated pyrazoles has been developed which relies on the cyclization of hydrazine dianions with ethyl perfluorocarboxylates. The pyrazoles prepared were evaluated as potential inhibitors of alkaline phosphatases, namely human tissue non-specific alkaline phosphatase (h-TNAP) and tissue specific intestinal alkaline phosphatase (IAP). Most pyrazole derivatives inhibited h-IAP more markedly than h-TNAP and had minor effects on nucleotide pyrophosphatase/phosphodiesterases. Therefore, the compounds appear as potential selective inhibitors of h-IAP.

 Please wait while we load your content...
Please wait while we load your content...