Oligoethylene glycol-substituted aza-BODIPY dyes as red emitting ER-probes†
Abstract
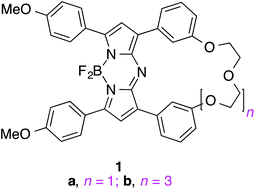
This study features aza-BODIPY (BF2-chelated azadipyrromethene) dyes with two aromatic substituents linked by oligoethylene glycol fragments to increase hydrophilicity of aza-BODIPY for applications in intracellular imaging. To prepare these, two chalcones were attached α,ω onto oligoethylene glycol fragments, then reacted with nitromethane anion. Conjugate addition products from this reaction were then subjected to typical conditions for synthesis of aza-BODIPY dyes (NH4OAc, nBuOH, 120 °C); formation of boracycles in this reaction was concomitant with creation of macrocycles containing the oligoethylene glycol fragments. Similar dyes with acyclic oligoelythene glycol substituents in the same position were used to compare the efficiencies of the intra- and inter-molecular aza-BODIPY forming reactions, and the characteristics of the products. All the fluors with oligoethylene glycol fragments, i.e. cyclic or acyclic, localized in the endoplasmic reticulum of a fibroblast cell line (WEHI-13VAR), the human pancreatic cancer cell line (PANC-1, rough ER predominates) and human liver cancer cell line (HepG2, smooth ER prevalent). These fluors are potentially useful for near IR (λmax emis at 730 nm) ER staining probes.

 Please wait while we load your content...
Please wait while we load your content...