Truce–Smiles rearrangement of substituted phenyl ethers†
Abstract
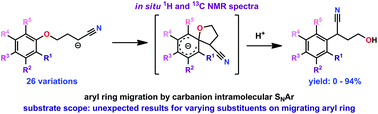
The requirement of aryl ring activation by strong-electron withdrawing substituents in substrates for the intramolecular nucleophilic aromatic substitution reaction known as the Truce–Smiles rearrangement was examined. Preliminary mechanistic experiments support the SNAr mechanism, including 1H and 13C NMR spectra of a Meisenheimer intermediate formed in situ. The rearrangement was generally observed to be successful for substrates with strong electron withdrawing substituents, such as nitro-, cyano-, and benzoyl- functional groups, but also for those with multiple, weakly electron withdrawing substituents, such as chloro- and bromo-functional groups. These results lend further clarification to the effect of aryl substituents in this type of SNAr reaction. Additionally, the survey revealed several tandem cyclization and/or elimination reactions accessed by certain substrates.

 Please wait while we load your content...
Please wait while we load your content...