Regioselective Suzuki couplings of non-symmetric dibromobenzenes: alkenes as regiochemical control elements†
Abstract
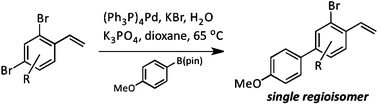
The regiochemical outcome of Suzuki couplings of non-symmetric dibromobenzenes is investigated. Selectivities are dependent on the proximity of the bromine atom to alkene substituents, not on steric or electronic effects. Extension to a one-pot three-component Suzuki reaction leads to efficient terphenyl syntheses.

 Please wait while we load your content...
Please wait while we load your content...