Intramolecular oxidative coupling: I2/TBHP/NaN3-mediated synthesis of benzofuran derivatives†
Abstract
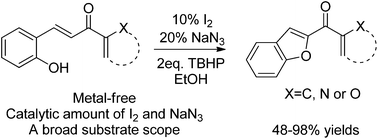
A novel intramolecular oxidative coupling reaction has been established to prepare benzofuran derivatives via direct C(sp2)–H functionalization for the formation of C–O bond. This transformation is mediated by I2/TBHP/NaN3 under metal-free conditions and a catalytic amount of NaN3 plays a crucial role in the reaction. Furthermore, the reaction tolerates a broad substrate scope with average to excellent yields.

 Please wait while we load your content...
Please wait while we load your content...