Copper(ii)-catalyzed highly regio- and stereo-selective hydrosilylation of unactivated internal alkynes with silylborate in water†
Abstract
The highly regio- and stereoselective hydrosilylation of internal alkynes with silylborate catalyzed by Cu(OTf)2 with 1,10-phenanthroline as the ligand in the presence of Cs2CO3 in water is developed. This protocol was applied efficiently in the aqueous synthesis of multi-substituted vinylsilanes.
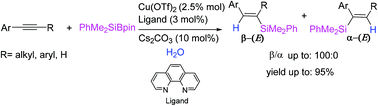

 Please wait while we load your content...
Please wait while we load your content...