Stereo-controlled synthesis of polyheterocycles via the diene-transmissive hetero-Diels–Alder reaction of β,γ-unsaturated α-keto esters†
Abstract
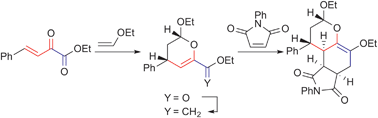
We describe the stereoselective synthesis of polyring-fused heterocyclic compounds based on diene-transmissive hetero-Diels–Alder reactions utilizing β,γ-unsaturated α-keto esters. This protocol involves the initial endo- or exo-selective Diels–Alder (DA) reactions with electron-rich dienophiles, methylenation of the ester carbonyl groups with the Tebbe reagent, and a stereoselective second DA reaction with electron-deficient dienophiles. The use of enantioselective DA reactions in the initial reaction enables access to chiral polyring-fused heterocyclic compounds with multiple chiral centres.

 Please wait while we load your content...
Please wait while we load your content...