Azetidine- and N-carboxylic azetidine-iminosugars as amyloglucosidase inhibitors: synthesis, glycosidase inhibitory activity and molecular docking studies†
Abstract
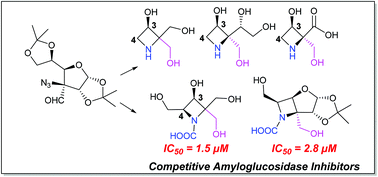
A simple strategy for the synthesis of hitherto unknown azetidine iminosugars 2a–2c and N-carboxylic azetidine iminosugar 2d has been reported. The methodology involves the conversion of 1,2:5,6-di-O-isopropylidene-3-oxo-α-D-glucofuranose 3 to 3-azido-3-deoxy-3-C-(formyl)-1,2:5,6-di-O-isopropylidene-α-D-glucofuranose 5 using the Jocic–Reeve and Corey–Link approaches. Compound 5 was transformed to 5-OTs 10/5-OMs 19 derivatives that on intramolecular nucleophilic displacement with in situ generated 3-amino functionality afforded the key azetidine ring skeletons 11 and 20, respectively. Hydrolysis of the 1,2-acetonide group and manipulation of the anomeric carbon in 12 provided azetidine iminosugars 2a–2c. In an attempt to synthesize azetidine iminosugars with an additional 4-hydroxymethyl group from 20, we encountered an interesting observation wherein the N-Cbz group in 20 hydrolyzed to the N-COOH functionality under TFA : H2O conditions that gave access for the synthesis of N-carboxylic azetidine iminosugar 2d. The glycosidase inhibitory activity of 2a–2d and intermediates 2e–f was studied with various glycosidases and was compared with Miglitol and 1-deoxynojirimycin (DNJ). Azetidine iminosugars 2 were found to inhibit amyloglucosidase with competitive type inhibition, amongst which 2d was found to be more active than Miglitol and DNJ. These results were substantiated by in silico molecular docking studies.

 Please wait while we load your content...
Please wait while we load your content...