A new method for the synthesis of difluoromethyl enol ethers by O-difluoromethylation of 1,3-diones with ClCF2CO2Et†
Abstract
A facile, efficient, single-step protocol for the synthesis of difluoromethyl enol ether derivatives by O-difluoromethylation of 1,3-diones via in situ generation of difluorocarbene from ClCF2CO2Et has been developed. The functional group tolerance, scalability of the reaction, and mild reaction conditions make it an attractive protocol for the synthesis of biologically relevant difluoromethyl ethers of interest to the pharmaceutical and agrochemical industries.
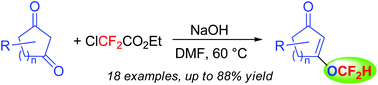

 Please wait while we load your content...
Please wait while we load your content...