Synthesis of phenstatin/isocombretastatin–chalcone conjugates as potent tubulin polymerization inhibitors and mitochondrial apoptotic inducers†
Abstract
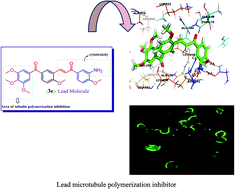
A series of phenstatin/isocombretastatin–chalcones were synthesized and screened for their cytotoxic activity against various human cancer cell lines. Some representative compounds exhibited significant antiproliferative activity against a panel of sixty human cancer cell lines of the NCI, with GI50 values in the range of 0.11 to 19.0 μM. Three compounds (3b, 3c and 3e) showed a broad spectrum of antiproliferative efficacy on most of the cell lines in the sub-micromolar range. In addition, all the synthesized compounds (3a–l and 4a–l) displayed moderate to excellent cytotoxicity against breast cancer cells such as MCF-7 and MDA-MB-231 with IC50 values in the range of 0.5 to 19.9 μM. Moreover, the tubulin polymerization assay and immunofluorescence analysis results suggest that some of these compounds like 3c and 3e exhibited significant inhibitory effect on the tubulin assembly with an IC50 value of 0.8 μM and 0.6 μM respectively. A competitive binding assay suggested that these compounds bind at the colchicine-binding site of tubulin. A cell cycle assay revealed that these compounds arrest at the G2/M phase and lead to apoptotic cell death. Furthermore, this was confirmed by Hoechst 33258 staining, activation of caspase 9, DNA fragmentation, Annexin V-FITC and mitochondrial membrane depolarization. Molecular docking studies indicated that compounds like 3e occupy the colchicine binding site of tubulin.

 Please wait while we load your content...
Please wait while we load your content...