Asymmetric Michael addition of α-fluoro-α-nitro esters to nitroolefins: towards synthesis of α-fluoro-α-substituted amino acids†
Abstract
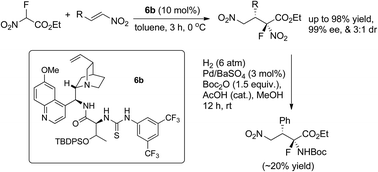
α-Fluoro-α-nitro esters were used as reaction partners in Michael addition to nitroalkenes, and the products were obtained in excellent chemical yields and with high enantioselectivities. Moreover, α-fluoro-α-amino ester with a quaternary α-carbon was prepared for the first time.

 Please wait while we load your content...
Please wait while we load your content...