Tetrahydrofuranyl and tetrahydropyranyl protection of amino acid side-chains enables synthesis of a hydroxamate-containing aminoacylated tRNA†
Abstract
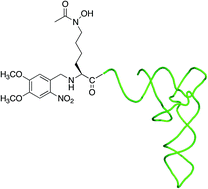
The ability to specifically engineer metal binding sites into target proteins has far-reaching consequences ranging from the development of new biocatalysts and imaging reagents to the production of proteins with increased stability. We report the efficient tRNA-mediated incorporation of the hydroxamate containing amino acid, Nε-acetyl-Nε-hydroxy-L-lysine, into a transcription factor (TFIIIA). Because this amino acid is compact, hydrophilic, and uncharged at physiological pH, it should have little or no effect on protein folding or solubility. The Nε-hydroxy group of the hydroxamate is refractory to photodeprotection and required the identification of reagents for O-protection that are compatible with the synthesis of acylated tRNA. Tetrahydrofuranyl and tetrahydropyranyl O-protecting groups can be removed using mild acid conditions and allowed for an orthogonal protection strategy in which deprotection of the amino acid side chain precedes ligation of an acylated dinucleotide to a truncated suppressor tRNA. These protecting groups will provide a valuable alternative for O-protection, especially in cases where photodeprotection cannot be used.

 Please wait while we load your content...
Please wait while we load your content...