Stereodivergent synthesis of the LFA-1 antagonist BIRT-377 by porcine liver esterase desymmetrization and Curtius rearrangement†
Abstract
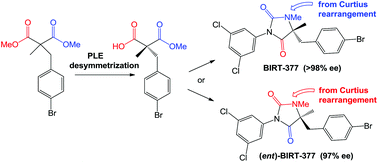
The LFA-1 inhibitor and leukocyte adhesion suppressor BIRT-377 was prepared in high enantiomeric excess by desymmetrization of dimethyl 2-p-bromobenzyl-2-methylmalonate, followed by condensation of the resulting carboxylic acid with 3,5-dichloroaniline, saponification of the remaining ester and Curtius rearrangement as the key steps. When Curtius rearrangement preceded the condensation step, (ent)-BIRT-377 was similarly obtained in high ee.

 Please wait while we load your content...
Please wait while we load your content...