Irreversible covalent modification of type I dehydroquinase with a stable Schiff base†
Abstract
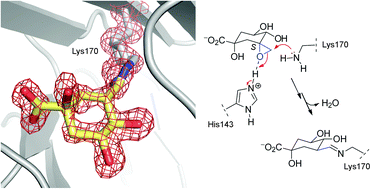
The irreversible inhibition of type I dehydroquinase (DHQ1), the third enzyme of the shikimic acid pathway, is investigated by structural, biochemical and computational studies. Two epoxides, which are mimetics of the natural substrate, were designed as irreversible inhibitors of the DHQ1 enzyme and to study the binding requirements of the linkage to the enzyme. The epoxide with the S configuration caused the covalent modification of the protein whereas no reaction was obtained with its epimer. The first crystal structure of DHQ1 from Salmonella typhi covalently modified by the S epoxide, which is reported at 1.4 Å, revealed that the modified ligand is surprisingly covalently attached to the essential Lys170 by the formation of a stable Schiff base. The experimental and molecular dynamics simulation studies reported here highlight the huge importance of the conformation of the C3 carbon of the ligand for covalent linkage to this type of aldolase I enzyme, revealed the key role played by the essential His143 as a Lewis acid in this process and show the need for a neatly closed active site for catalysis.
 Please wait while we load your content...
Please wait while we load your content...