Effects of crystallographic facet-specific peptide adsorption along single ZnO nanorods on the characteristic fluorescence intensification on nanorod ends (FINE) phenomenon
Abstract
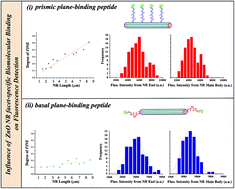
The precise effect of crystallographically discriminating biomolecular adsorption on the fluorescence intensification profiles of individual zinc oxide nanorod (ZnO NR) platforms was elucidated in this study by employing peptide binding epitopes biased towards particular ZnO crystal surfaces and isolating the peptides on given crystalline facets of ZnO NRs. Subsequently, the fluorescence emission profiles of the preferentially bound peptide cases on the basal versus prismic planes of ZnO NRs were carefully evaluated both experimentally and via computer simulations. The phenomenon of fluorescence intensification on NR ends (FINE) was persistently observed on the individual ZnO NR platforms, regardless of the location of the bound peptides. In contrast to the consistent occurrence of FINE, the degree and magnitude of FINE were largely influenced by the discriminatory peptide adsorption to different ZnO NR facets. The temporal stability of the fluorescence signal was also greatly affected by the selectively located peptides on the ZnO NR crystal when spatially resolved on different NR facets. Similarities and differences in the spatial and temporal fluorescence signal of the crystalline NR facet-specific versus -nonspecific biomolecular adsorption events were then compared. To further illuminate the basis of our experimental findings, we also performed finite-difference-time-domain (FDTD) calculations and examined the different degrees of FINE by modelling the biased peptide adsorption cases. Our multifaceted efforts, providing combined insight into the spatial and temporal characteristics of the biomolecular fluorescence signal characteristically governed by the biomolecular location on the specific NR facets, will be valuable for novel applications and accurate signal interpretation of ZnO NR-based biosensors in many rapidly growing, highly miniaturized biodetection configurations.

 Please wait while we load your content...
Please wait while we load your content...