One-phase controlled synthesis of Au25 nanospheres and nanorods from 1.3 nm Au : PPh3 nanoparticles: the ligand effects
Abstract
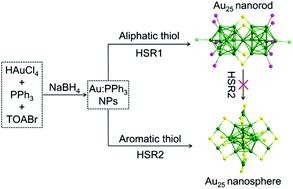
We report the controlled synthesis of [Au25(PPh3)10(SR1)5X2]2+ nanorods (H-SR1: alkyl thiol, H-SC2H4Ph and H-S(n-C6H13)) and Au25(SR2)18 nanospheres (H-SR2: aromatic thiol, H-SPh and H-SNap) under the one-phase thiol etching reaction of the polydisperse Aun(PPh3)m parent-particles (core diameter: 1.3 ± 0.4 nm, 20 < n < 50). These as-obtained gold nanoclusters are identified by UV-vis spectroscopy and matrix-assisted laser desorption ionization mass spectrometry. Furthermore, the conversion process, from Aun(PPh3)m nanoparticles to Au25(SNap)18 nanospheres, is monitored by UV-vis spectroscopy. It is observed that the Au25(PPh3)10(SR1)5X2 nanorods cannot convert to Au25(SR)18 nanospheres in the presence of excess thiol (both the alkyl and aromatic thiol) even under thermal conditions (e.g., 55 and 80 °C), indicating that both the Au25 nanorods and nanospheres are in a stable state during the alkyl and aromatic thiol etching reactions, respectively. The two different conversion pathways (i.e., to Au25(PPh3)10(SR1)5X2 nanorods and Au25(SR2)18 nanospheres) mainly are attributed to the different electronic properties and the steric effects of the alkyl and aromatic thiol ligands. The significant ligand effect also is observed in catalytic CO oxidation. The Au25(SC2H4Ph)18/CeO2 catalyst shows catalytic activity at 80 °C and reaches up to 80.7% and 98.5% (based on CO conversion) at 100 and 150 °C, while Au25(SNap)18/CeO2 and Au25(PPh3)10(SC2H4Ph)5X2/CeO2 give rise to a low activity at 100 °C with only 3.3% and 10.2% CO conversion and 98.0% and 94.6% at 150 °C.

 Please wait while we load your content...
Please wait while we load your content...