Saccharin salts of biologically active hydrazone derivatives†
Abstract
The crystal structures of two saccharin salts with derivatives of an anti-tubercular drug isoniazid, namely vanillin isoniazid saccharinate (salt I) and salinazid saccharinate (salt II), were obtained in a X-ray diffraction experiment. The pattern of intermolecular interactions in the crystals was quantified by solid-state DFT followed by the Bader analysis of periodic electron density. It was established that ca. 42% of lattice energy is contributed by C–H⋯O contacts, while conventional hydrogen bonds have only ca. 28%. Salt I was found to show a 12-fold aqueous solubility improvement compared to pure API, whereas salt II is approximately 20 times more soluble than the starting salinazid. The standard thermodynamic functions of the salt formation were determined. The Gibbs energy change of the process was found to be negative, indicating that the formation of the salts from individual components is a spontaneous process. The most significant contribution to the Gibbs energy is provided by the enthalpy term, while the entropy change of the process has a negative value, introducing a positive contribution to 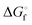 .
.

 Please wait while we load your content...
Please wait while we load your content...