Synthesis and optical properties of a new triphenylamine-p-phenylenevinylene-small molecule with applications in high open-circuit voltage organic solar cells†
Abstract
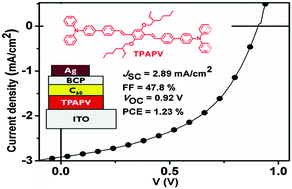
A new oligomer-type para-phenylenevinylene end-capped with triphenylamine groups (TPAPV) was synthesised and its optical properties thoroughly investigated. Fluorescence quenching studies showed that an important fraction of the photogenerated excitons formed in TPAPV is quenched by [6,6]-phenyl-C61-butyric acid methyl ester (PC61BM) in blend films, therefore indicating efficient exciton dissociation and potential applications in organic photovoltaic (PV) cells. Planar heterojunction organic photovoltaic cells containing TPAPV as the electron-donor were fabricated and devices with structure ITO/TPAPV(6 nm)/C60(50 nm)/BCP(10 nm)/Ag exhibited an open-circuit voltage (VOC), short-circuit current (JSC), fill factor (FF), and power-conversion efficiency (PCE) of 0.92 V, 2.9 mA cm−2, 47.8%, and 1.23%, respectively.

 Please wait while we load your content...
Please wait while we load your content...