Experimental and theoretical investigation of tetra-oxidized terarylenes with high-contrast fluorescence switching†
Abstract
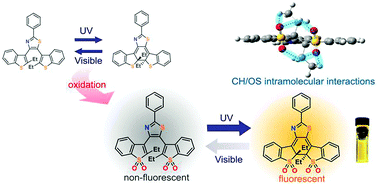
Tetra-oxidized terarylenes having ethyl groups at the reactive carbon atoms are synthesized and their photophysical properties are studied. Their closed-ring isomers exhibit much lower cycloreversion quantum yields and relatively high fluorescence quantum yields. Introducing ethyl groups at the reactive carbon atoms provides an improvement in the fluorescence quantum yields by the intramolecular hydrogen bonding in the closed-ring isomers to reduce the non-radiative decay processes coupled with molecular motions. Quantum chemical calculations were carried out to study their geometrical and optical properties, which agree well with the experimental findings.

 Please wait while we load your content...
Please wait while we load your content...