The DIONESUS algorithm provides scalable and accurate reconstruction of dynamic phosphoproteomic networks to reveal new drug targets†
Abstract
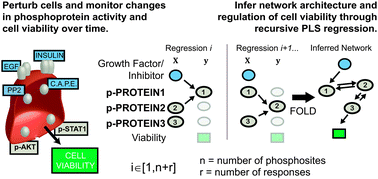
Many drug candidates fail in clinical trials due to an incomplete understanding of how small-molecule perturbations affect cell phenotype. Cellular responses can be non-intuitive due to systems-level properties such as redundant pathways caused by co-activation of multiple receptor tyrosine kinases. We therefore created a scalable algorithm, DIONESUS, based on partial least squares regression with variable selection to reconstruct a cellular signaling network in a human carcinoma cell line driven by EGFR overexpression. We perturbed the cells with 26 diverse growth factors and/or small molecules chosen to activate or inhibit specific subsets of receptor tyrosine kinases. We then quantified the abundance of 60 phosphosites at four time points using a modified microwestern array, a high-confidence assay of protein abundance and modification. DIONESUS, after being validated using three in silico networks, was applied to connect perturbations, phosphorylation, and cell phenotype from the high-confidence, microwestern dataset. We identified enhancement of STAT1 activity as a potential strategy to treat EGFR-hyperactive cancers and PTEN as a target of the antioxidant, N-acetylcysteine. Quantification of the relationship between drug dosage and cell viability in a panel of triple-negative breast cancer cell lines validated proposed therapeutic strategies.

 Please wait while we load your content...
Please wait while we load your content...